In the statement made by the World Health Organization (WHO), there are warnings that one should not be ‘overly optimistic’ by expressing the hope that hundreds of millions of doses of coronavirus vaccines will be produced before the end of the year.
WHO Chief Scientist Soumya Swaminathan said yesterday that WHO hopes that hundreds of millions of doses of coronavirus vaccine will be produced before the end of the year and 2 billion by the end of 2021. Swaminathan added that WHO has developed plans for who should receive the first doses of any vaccine to be approved. According to Reuters, people who are at the forefront in the fight against coronavirus, medical staff, and those who are most at risk for age or other diseases, such as prisons or nursing homes, who work or reside in areas where the virus is easily transmitted, will be given priority over vaccination.
“I am hopeful and optimistic about this,” said Swaminathan. But developing a vaccine is a complex task, with a lot of uncertainty. The good thing is that there are a lot of vaccine studies and work approaches. Therefore, if one or two vaccines fail, we should not lose hope or give up. ” Describing the hope of producing about 2 billion doses from three different vaccines next year as ‘ambiguous’, Swaminathan said that the genetic analysis data collected so far shows that the new type of coronavirus has not yet been mutated.
Competition is heating up
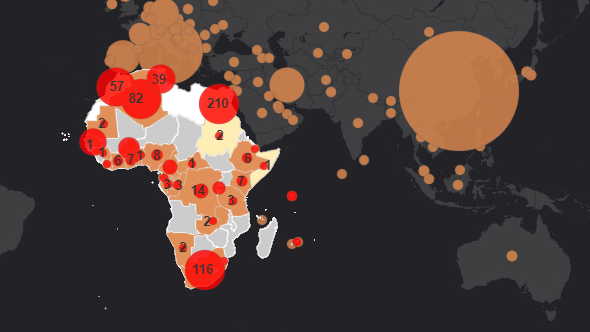
Competition between laboratories, pharmaceutical companies and universities has intensified over the past few weeks to develop an effective Covid-19 vaccine. According to AFP’s report, on June 16, WHO reported that 11 different vaccine studies worldwide are at the clinical stage. Five of these studies are conducted on people in China, where a second wave of outbreaks erupted in Beijing. China, trying to be the first in a race to find a vaccine, has speeded up the procedures in this regard.
In clinical studies on vaccination, the ‘first stage’ is passed, in which product safety is verified. Then, the ‘second stage’ is reached, where the event is evaluated. Only partial results have been published so far, and these have been identified as ‘encouraging’.
Oxford University’s study in collaboration with AstraZeneca and the study of the Chinese pharmaceutical company called the Chinese Academy of Military Medical Sciences and CanSino Biologics are among the advanced research. WHO reported that 128 vaccine projects are taking place at the preclinical stage. London Hygiene and Tropical Medicine School has 194 different vaccine projects, of which 17 are in trial phase.
Hydroxychloroquine controversy
In a statement yesterday, WHO announced that it is aware of ongoing tests about the hydroxychloroquine drug to find out how much it has helped to combat coronavirus. This drug, which has been used for the treatment of malaria and rheumatoid arthritis patients for many years, has led to political and scientific controversy, and then its use in hospitals has been abolished. After research showed that the drug in question had no effect in reducing the mortality rate, WHO decided to stop using the drug on hospital coronavirus patients Wednesday. On the other hand, WHO Chief Scientist Swaminathan said at the meeting via video conference, “The last words about the use of the hydroxychloroquine drug on Kovid-19 cases were not said. As a matter of fact, some good and big tests are going on. We hope that they will be completed so that we have sufficient evidence to help patients with whom the drug will benefit. ”
Leading world leaders, such as U.S. President Donald Trump, encouraged the use of the hydroxychloroquine drug in the treatment of Kovid-19 patients. The drug was used randomly in many clinical tests, counting as the best standard of clinical trials. However, WHO said the evidence pushed the tests to stop. “It is clear that hydroxychloroquine has no effect on the disease in relation to the deaths of Kovid-19 patients in hospitals,” said Swaminathan, a pediatrician. We now know for sure. However, we do not yet know if it played any role in the early stage of the infection. ”
The hydroxychloroquine drug was tested in random trials on healthcare workers and others who were exposed to the virus in general too much.